
In another first, an instrument inside NASA’s Perseverance rover has made oxygen out of carbon dioxide sucked in from the atmosphere of Mars, officials said Wednesday. The technology could help future astronauts “live off the land” by generating their own rocket fuel and breathing air.
The milestone for the Perseverance rover’s Mars Oxygen In-Situ Resource Utilization Experiment, one of the six-wheeled robot’s seven instruments. The rover’s other experiments study the Martian environment, while MOXIE is a pure technology demonstration.
NASA said Wednesday the MOXIE instrument — about the size of a toaster oven — succeeded in generating oxygen on Mars. The instrument works by ingesting carbon dioxide, which makes up about 96% of the Martian atmosphere, and separating one of the molecules’ oxygen atoms from a second oxygen atom and carbon.
“This is a critical first step at converting carbon dioxide to oxygen on Mars,” said Jim Reuter, associate administrator for NASA’s space technology mission directorate, which partnered with NASA’s human spaceflight division to develop the MOXIE instrument. “MOXIE has more work to do, but the results from this technology demonstration are full of promise as we move toward our goal of one day seeing humans on Mars.”
The instrument emits carbon monoxide, the waste product left over after the conversion, back into the Martian atmosphere. Future missions, including astronaut explorers, could use a similar process to generate tons of oxygen to use as rocket propellant and breathable air.
“Oxygen isn’t just the stuff we breathe,” Reuter said. “Rocket propellant depends on oxygen, and future explorers will depend on producing propellant on Mars to make the trip home.”
NASA said the MOXIE instrument completed its first trial run Tuesday, April 20. Over the course of about an hour, the instrument produced 5.4 grams of oxygen, enough for an astronaut to breathe for about 10 minutes, according to NASA. The agency said MOXIE is designed to generate up to 10 grams of oxygen per hour.
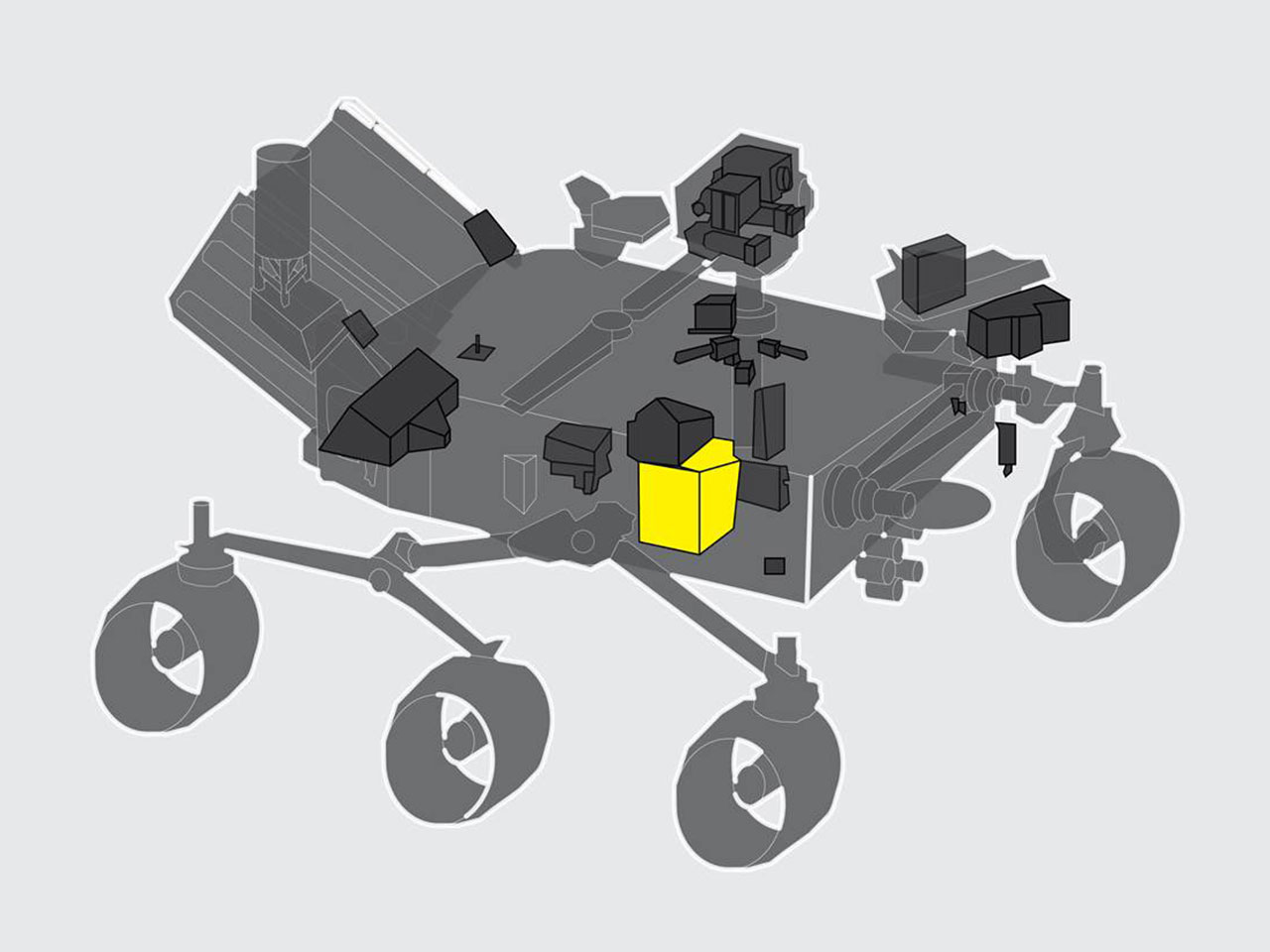
In order to strip oxygen out of carbon dioxide molecules, MOXIE heats the gas to a temperature near 1,470 degrees Fahrenheit (800 Celsius), according to NASA.
“To accommodate this, the MOXIE unit is made with heat-tolerant materials,” NASA said. “These include 3D-printed nickel alloy parts, which heat and cool the gases flowing through it, and a lightweight aerogel that helps hold in the heat. A thin gold coating on the outside of MOXIE reflects infrared heat, keeping it from radiating outward and potentially damaging other parts of Perseverance.”
A future human expedition to Mars would need a scaled-up oxygen generation unit. A rocket powerful enough to launch astronauts off the surface of Mars would need about 15,000 pounds (7 metric tons) of fuel and 55,000 pounds (25 metric tons) of oxygen, NASA said.
The astronauts would need less oxygen to breathe. Four astronauts might require about a metric ton of breathable oxygen in a year, said Michael Hecht, the MOXIE instrument’s principal investigator at the Massachusetts Institute of Technology’s Haystack Observatory.
The MOXIE instrument is the first experiment on Mars to prove out technologies for in situ resource utilization, where missions rely on natural materials on other planets to live off the land. Other resources that future astronauts could use include rocks and soil to help create structures, or water from ice deposits.
NASA plans nine more oxygen generation runs on the MOXIE instrument over the next two years. The experiments will test the instrument’s performance at different temperature settings, and measure how MOXIE works at different times of day on Mars.
Email the author.
Follow Stephen Clark on Twitter: @StephenClark1.



